(JollofNews) As I complete my ward round in a London Teaching Hospital with a visit to the 7 year old boy who has already lost both lower limbs due to meningococcal disease and who is staring at his black, necrotic fingers, I remind myself that this child was actually vaccinated as a baby with a vaccine against Meningococcus Type C (MenC).
This vaccine was introduced into the UK routine immunization schedule in 1999, and is now offered to every child in the country in infancy with a booster in adolescence. The vaccine and its resulting direct protection and indirect effects on herd immunity have since reduced the numbers of children suffering from meningitis or septicaemia caused by MenC in the UK from an annual average of 1000 to under 10 cases.
As paediatricians, we have seen the cases disappear from our intensive care units, and bacterial meningitis in young children overall is now rare in the UK, thanks to the combined effects of vaccines introduced over the last 20 years against Haemophilus influenza, Pneumococcus and MenC. A vaccine against Meningococcus Type B (MenB), the other relative of the deadly bacterium, was introduced in 2015 but currently its use remains restricted to infants under the age of 1, primarily on grounds of cost effectiveness deliberations.
A vaccine against Meningococcus Type B (MenB), the other relative of the deadly bacterium, was introduced in 2015 but currently its use remains restricted to infants under the age of 1, primarily on grounds of cost effectiveness deliberations.
On Monday, April 25th a petition initiated by families affected by recent cases of MenB and supported by the UK meningitis charities will be debated in the Houses of Parliament. This largest ever petition asks to widen access to the new MenB vaccine and has attracted over 820 000 signatures to date. The petition also questions the current model employed to evaluate the cost effectiveness of vaccines, the main determinant for the decision whether new vaccines are introduced in the UK. Even with vaccines known to prevent serious illness, most decisions boil down to costs, largely determined by the market prices set by the vaccine industry.
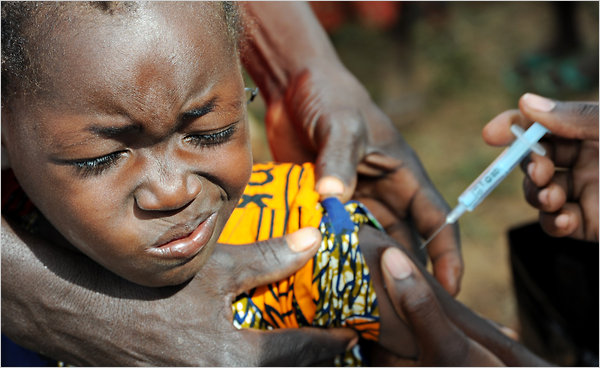
This is disappointing news for families trying to protect their vulnerable youngsters,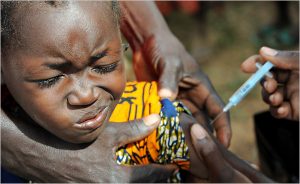 and maybe we could consider for a moment recent examples from resource-poorer settings, where live-saving vaccines against meningitis have recently been introduced:
and maybe we could consider for a moment recent examples from resource-poorer settings, where live-saving vaccines against meningitis have recently been introduced:
In 2009 a new vaccine against meningitis caused by Meningocococcus type A (MenA) was licensed and WHO pre-qualified in 2010. MenAfriVac was developed and produced through a partnership called the Meningitis Vaccine Project (MVP), which included the WHO, PATH, NIH and the Serum Institute of India Ltd. who had a clear mandate to develop a vaccine against an equally deadly form of meningitis, known to periodically sweep through entire areas of sub-saharan Africa and killing thousands of individuals during such epidemics.
The subsequent development of MenAfriVac is a successful example of federal laboratories and universities licensing their technologies to organizations other than traditional pharmaceutical and biotech companies, and achieved product commercialization and thus public utilization of their research. MenAfriVac was developed and licensed within 10 years and is now part of the WHO recommended vaccination schedule in countries of the meningitis belt. By 2020, the vaccine is expected to protect more than 400 million people, preventing 1 million cases of meningitis A, 150,000 deaths, and 250,000 cases of severe disability. It costs just US$ 0.40 per dose.
The second example relates to more efficient use of an already licensed product:
In April 2016, a new preparation of Prevenar 13, a 13-valent pneumococcal vaccine made by Pfizer was approved by the European Medicines Agency (EMA). Instead of just 1 dose per vial, this multi-dose vial contains 4 doses, which will significantly lower its distribution costs for low-and middle- income countries and thereby make this vaccine more affordable and accessible to more children.
The development of this vaccine preparation was driven forward by GAVI, the Vaccine Alliance, and once WHO prequalification is achieved, the vaccine will be available for global use by United Nations agencies and countries worldwide that require WHO prequalification under the Advance Market Commitment program in early 2017.
These two examples of successful public-private programs resulted from a commitment to global health and have resulted in more affordable vaccines.
As parliament debates the affordability of potentially life saving vaccines in a comparatively highly resourced setting such as the UK, maybe such new models of partnership could serve as inspiration. In the meantime, we as clinicians and researchers need to answer the question why the young boy on my ward failed to be protected despite having received his recommended vaccine at the right time.
Beate Kampmann is Professor of Paediatric Infection & Immunity at Imperial College London and Director of the Centre for International Child Health. She also holds an appointment at the MRC Unit in The Gambia, West Africa where she leads the vaccine research.
This article was first posted on the BMJ Publishing Group website on 25 April, 2016